- F Valence Electrons
- F Has __ Valence Electrons
- F Valence Electrons Number
- F Element Valence Electrons
- Valence Electrons In Each Element

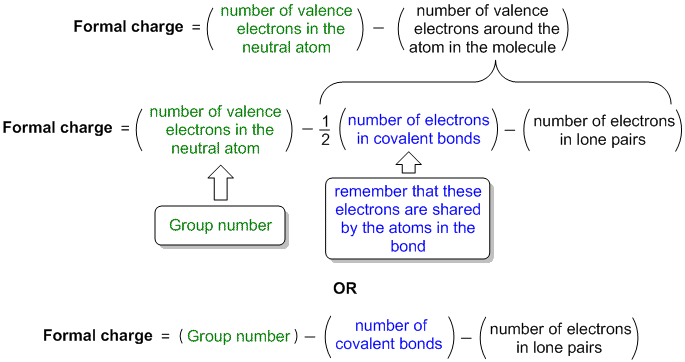
- Valence electrons are those outermost electrons that dictate the properties of the atom and are involved in chemical bonding. The valence electrons are those in the outermost s sublevel and any unfilled sublevels. The number of valence electrons in an atom equals the group number of the atom.
- In this video we will write the electron configuration for F-, the Fluoride ion. We’ll also look at why Fluorine forms a 1- ion and how the electron configur.
- See full list on wikihow.com.
- These electrons, called the valence electrons, are the most loosely held and interact with those in other atoms to form chemical bonds. The type of orbital (s, p, d, or f) that the valence electrons reside in is a function of the elements' position in the periodic table.
Waiting for answer This question has not been answered yet. You can hire a professional tutor to get the answer.
Fluorine has 7 . Bootable mac os x usb for pc.
Valence electrons are those electrons which are present in the outermost shell of an atom.
Fluorine has atomic number 9. Atomic number is the number of protons present in the nucleus of an atom.
In every stable (Neutral) atom the number of electrons are equal to the number of protons.
Therefore, the number of electrons in an atom of fluorine are 9.
The electronic configuration of fluorine is: Convert avi to mp4 for mac.
E.C:- k - 2 , L-7
We can define valence electrons as electrons on an atom that are not present in the previous rare gas, ignoring filled d or f subshells. Many books published in the last 10 years use this definition. That d electrons may be valence electrons is also supported by the 18-Electron rule (at least to the extent that there is such a rule).

The last shell of fluorine has 7 electrons. Therefore there are 7 valence electrons in an atom of fluorine.
GET YOUR EXPERT ANSWER ON STUDYDADDY
Post your own question
and get a custom answer

- Q: What is the difference between electronegativity and electron affinity?
- Q: How would you rearrange the Henderson–Hasselbalch equation to find out [a/ha] from pH=pKa + log[a/ha] ?
- Q: Why does ice float instead of sink?
- Q: What is the electron configuration, orbital diagram, and noble gas notation of potassium?
- Q: Are acid-base reactions always double displacement?
- Q: cobalt (II) sulfate reacts with lead (II) nitrate farming cobalt (II) nitrate and lead (II) sulfate
- Q: A book describes a reaction as follows: Methane b
F Valence Electrons
How many valence electrons does fluorine have?
Fluorine has 7 .
Valence electrons are those electrons which are present in the outermost shell of an atom.
Fluorine has atomic number 9. Atomic number is the number of protons present in the nucleus of an atom.
In every stable (Neutral) atom the number of electrons are equal to the number of protons.
Therefore, the number of electrons in an atom of fluorine are 9.
The electronic configuration of fluorine is:
F Has __ Valence Electrons
E.C:- k – 2 , L-7
F Valence Electrons Number
The last shell of fluorine has 7 electrons. Therefore there are 7 valence electrons in an atom of fluorine.
F Element Valence Electrons
Valence Electrons In Each Element
Related

